Is the quadratus lumborum really that clinically important?
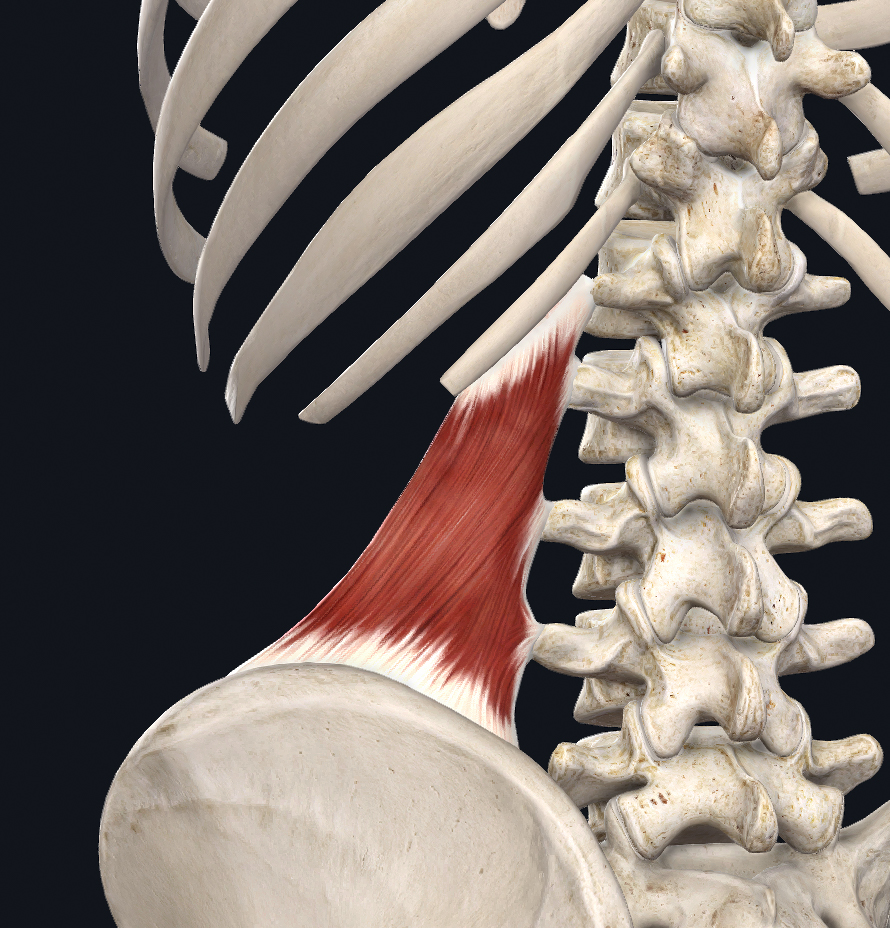 The quadratus lumborum muscle (QL) is one of the most commonly mentioned muscles in discussions around back pain, but does it really deserve all of that credit? Is it the culprit or the victim? Like the deltoid, the QL receives a lot of referral from other muscles which can mislead the clinician into thinking it is the primary source of pain although its force exertion is poor compared to surrounding muscles. Since most skeletal muscles of the human body are directly linked by connective tissue, symptoms may develop in areas distant from the locus of dysfunction (Wilke et al, 2016).
The quadratus lumborum muscle (QL) is one of the most commonly mentioned muscles in discussions around back pain, but does it really deserve all of that credit? Is it the culprit or the victim? Like the deltoid, the QL receives a lot of referral from other muscles which can mislead the clinician into thinking it is the primary source of pain although its force exertion is poor compared to surrounding muscles. Since most skeletal muscles of the human body are directly linked by connective tissue, symptoms may develop in areas distant from the locus of dysfunction (Wilke et al, 2016).
What is FSN- AV?
Fu Subcutaneous Needling – American Version, also known as FSN-AV, is a new way to look at structural integration by incorporating the dynamics of fascia and its pulley systems within the body. By using a fascial needle, immediate changes can be seen (and felt) in structure, ROM, pain, and tissue density. Resting muscle tone may be significantly influenced by changes in fascial stiffness (Masi & Hannon, 2008). Looking at cellular physiology, the cytoskeletal remodeling of fibroblasts is thought to contribute to connective tissue tension. As fascia contains various sensory receptors for proprioception, nociception, and hormones, nociception is influenced by the state of the fascia (Nordez et al, 2017). Fu Subcutaneous Needling – American Version, unlike other therapies, targets the fascia directly, evoking a more significant change to the surrounding tissues both immediate and further along the fascial chain. With direct contact with the fascia and the manipulation of the needle, fibroblasts are stretched, collagen fibers are realigned and an alteration of densified tissue can be seen.
“The visco-elastic behavior of connective tissue is generally attributed to the material properties of the extracellular matrix rather than cellular activity. We have previously shown that fibroblasts within areolar connective tissue exhibit dynamic cytoskeletal remodeling within minutes in response to tissue stretch ex vivo and in vivo” (Langevin et al, 2011)
Significantly increased resting tissue tension can be seen when cytoskeletal dynamics are compromised by actomyosin contractility, due to Rho kinase inhibition. By changing shape, fibroblasts can dynamically modulate the viscoelastic behavior of areolar connective tissue through Rho-dependent cytoskeletal mechanisms.
Within the tensegrity model, cells are hard-wired to respond immediately to mechanical stress (Ingber, 1997), transmitted by physical stressors on tissues through stretch-sensitive ion channels and signaling molecules (known as mechanotransduction), transduced through integrins that directly influence the extracellular matrix. By remodeling their cytoskeleton, fibroblasts rapidly (within seconds to minutes) modulate the stiffness and viscosity of areolar connective tissue.
“The mechanical environment can be directly affected by the fibroblasts, thanks to their ability to control and modulate the extracellular matrix, which indirectly determines the function of the different systems dealing with the fascial continuum” (Bordoni & Zanier, 2015)
How does this relate to lower back pain and the QL?
A 2017 histological study at Goethe University Frankfurt demonstrated that the presence of nociceptive free nerve endings within the lumbodorsal fascia appear to exhibit morphological changes in patients with chronic low back pain. Exploring this further, changes in the density of fascial tissue may be sufficient to entrap nociceptive nerve endings and cause pain. One human study demonstrated that a single dose of nerve growth factor induced stronger mechanical hyperalgesia in fascia than muscle (Wu et al, 2009). Thoracolumbar fascia was also found to be highly sensitive to NGF-induced mechanical hyperalgesia, even more so than tibial fascia. These findings have important clinical implications and suggest that sensitization of fascia rather than muscle by NGF may be an important mechanism contributing to the pathophysiology of musculoskeletal pain, especially low back pain.

Various actions on the lumbar spine biomechanics have been attributed to the quadratus lumborum, but they have not been substantiated by quantitative data.
A 2008 study (Phillips et al) suggests that the magnitudes of the compression forces exerted by the quadratus lumborum on the lumbar spine, the extensor moment, and the lateral bending moment, were each no greater than 10 percent of those exerted by the erector spinae and the multifidi. During back extension, the QL exerts a force of 10Nm, compared to 100Nm and 150Nm of the erector spinae muscles and the multifidi. There is still no certainty that an abnormality of the QL is the primary source of back pain. The QL could potentially act as a crossroad of the forces exerted by the neighboring muscles, influencing the vectors of the different tensions produced.
Revisiting the 2017 study, fascial contraction and densification entrapping nociceptive nerve endings, we can further explore the fascial dynamics and the pulley systems of the fascial planes as the fascia glides through collagenous structures. According to Thomas Myers, there is an antagonistic relationship between, what he refers to as the superficial front and back lines. When the front line contracts or tightens, this will simply lead to taut stretching of the back line, tissue modification including viscoelastic changes to the fascia, and a decrease in nerve mobility. Changes in connective tissue behavior within the microenvironment around the nerve can lead to entrapments and lesions (Kagawa et al, 2021). Ergonomics and functional day-to-day movements and holding patterns must also play a role. A large percentage of the population can identify with spending long hours of the day sitting at a desk, driving in a car, sitting on the couch, looking down at the phone, etc. A less sedentary and more movement-aware person may engage in bicycling, running, rowing, etc. These daily activities all encourage shortening of the front line, tightening the back fascial line, and anteriorly rotating the pelvis.
Conclusion: Why Fu Subcutaneous Needling – American Version Reduces Lower Back Pain
Fu Subcutaneous Needling – American Version takes these dynamics into consideration through a big picture assessment by treating back pain and tension from the front of the body by releasing the line of dysfunction, allowing the back fascia to ease. The FSN-AV theory suggests that once the body regains stability, tension will be released. As our daily activities and functional holding patterns encourage a more concave posture, we can see a tightening of the abdominal muscles, diaphragm, and psoas, which not only refer pain to the lower back but directly share the same fascia along with the QL. It takes no more than just a few short minutes of manipulation of the FSN-AV needles for immediate fascial changes to be seen, to release tension within these pulley systems and achieve relief of pain, loosening of the QL and surrounding muscles as well as opening a window for further work with other modalities as an integrated treatment protocol for lower back pain.
If you are interested in getting certified in Fu Subcutaneous Needling – American Version register for a course Today.
Advanced Dry Needling Course
An advanced needling course. The Fu Subcutaneous Needling – American Version Course (FSN) is designed to provide students with a comprehensive introduction to fascial needling by areas of the body utilizing ROM assessment, treatment skills, and techniques specific to the field of myofascial pain and muscle dysfunction.
References:
Bordoni B, Zanier E. Understanding Fibroblasts in Order to Comprehend the Osteopathic Treatment of the Fascia. Evid Based Complement Alternat Med. 2015;2015:860934. doi: 10.1155/2015/860934. Epub 2015 Aug 19. PMID: 26357524; PMCID: PMC4556860.
Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575-99. doi: 10.1146/annurev.physiol.59.1.575. PMID: 9074778.
Kagawa E, Nimura A, Nasu H, Kato R, Akita K. Fibrous Connection Between Cervical Nerve and Zygapophysial Joint and Implication of the Cervical Spondylotic Radiculopathy: An Anatomic Cadaveric Study. Spine (Phila Pa 1976). 2021 Jul 1;46(13):E704-E709. doi: 10.1097/BRS.0000000000003895. PMID: 33337682.
Langevin HM, Bouffard NA, Fox JR, Palmer BM, Wu J, Iatridis JC, Barnes WD, Badger GJ, Howe AK. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J Cell Physiol. 2011 May;226(5):1166-75. doi: 10.1002/jcp.22442. PMID: 20945345; PMCID: PMC3053527.
Masi AT, Hannon JC. Human resting muscle tone (HRMT): narrative introduction and modern concepts. J Bodyw Mov Ther. 2008 Oct;12(4):320-32. doi: 10.1016/j.jbmt.2008.05.007. Epub 2008 Jul 7. PMID: 19083691.
Myers, T.W. (2021) Anatomy Trains: Myofascial Meridians for Manual Therapists & Movement Professionals. Edinburgh, Churchill Livingstone, 4 (4) pg. 55. Fig. 4.4
Nordez A, Gross R, Andrade R, Le Sant G, Freitas S, Ellis R, McNair PJ, Hug F. Non-Muscular Structures Can Limit the Maximal Joint Range of Motion during Stretching. Sports Med. 2017 Oct;47(10):1925-1929. doi: 10.1007/s40279-017-0703-5. PMID: 28255938.
Phillips S, Mercer S, Bogduk N. Anatomy and biomechanics of quadratus lumborum. Proc Inst Mech Eng H. 2008 Feb;222(2):151-9. doi: 10.1243/09544119JEIM266. PMID: 18441751.
Wilke J, Krause F, Vogt L, Banzer W. What Is Evidence-Based About Myofascial Chains: A Systematic Review. Arch Phys Med Rehabil. 2016 Mar;97(3):454-61. doi: 10.1016/j.apmr.2015.07.023. Epub 2015 Aug 14. PMID: 26281953.
Wilke J, Schleip R, Klingler W, Stecco C. The Lumbodorsal Fascia as a Potential Source of Low Back Pain: A Narrative Review. Biomed Res Int. 2017;2017:5349620. doi: 10.1155/2017/5349620. Epub 2017 May 11. PMID: 28584816; PMCID: PMC5444000.
Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology. 2009 Jan;110(1):140-9. doi: 10.1097/ALN.0b013e318190bc84. PMID: 19104181; PMCID: PMC2727137.