While the impact of dry needling (DN) has been established for various indications and body regions [1-21], it is not necessarily clear when or even whether dry needling of patients with systemic hypermobility is indicated. Hypermobile individuals may “create” muscle contractures, commonly known as “taut bands’ in the myofascial pain literature, to provide stability in otherwise unstable joints. It is conceivable that lowering the muscle tone of such contractures would increase joint instability, leading to an immediate decrease in function. Before deciding to use dry needling on a patient with hypermobile joints, a brief review follows, summarizing some of the main characteristics of hypermobility, Ehlers-Danlos Syndrome (EDS), and Hypermobility Spectrum Disorders (HSD).
Hypermobility
Joint mobility is a prerequisite to movement and motor development. Physiotherapists and other healthcare providers commonly measure joint range of motion against suggested norms required for movement efficiency [22]. When joint range deviates from these normative values, clinicians characterize the joint as either hypo- or hypermobile and offer therapy to address impairments in joint mobility and improve movement efficiency [23].
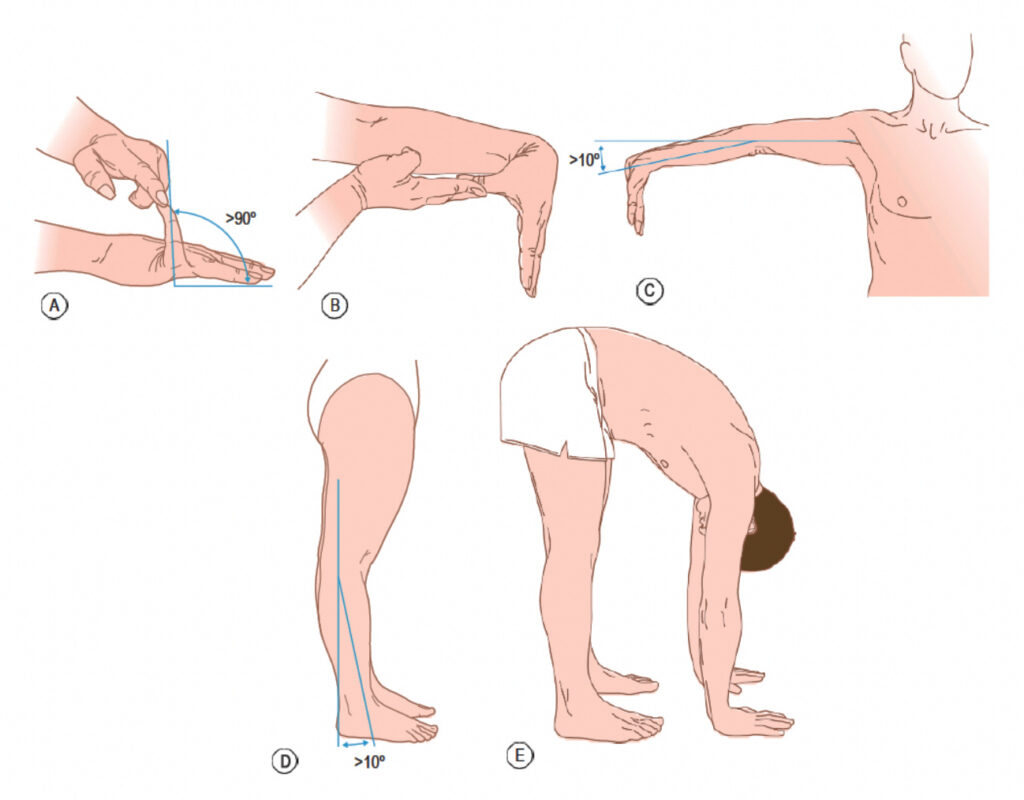 Hypermobility is quite common in patients, ranging from having one or two hypermobile joints to having generalized HSD or EDS [24, 25]. Hypermobility is defined as excessive joint movement within a normal plane of motion. Although in the clinic, the terms hypermobility, hyperlaxity, and hyperextensibility are often used interchangeably, the terms are not equal as the latter refers only to movement in abnormal planes [26].
Hypermobility is quite common in patients, ranging from having one or two hypermobile joints to having generalized HSD or EDS [24, 25]. Hypermobility is defined as excessive joint movement within a normal plane of motion. Although in the clinic, the terms hypermobility, hyperlaxity, and hyperextensibility are often used interchangeably, the terms are not equal as the latter refers only to movement in abnormal planes [26].
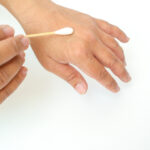
Hypermobility can be structural, constitutional, or hereditary, but it can also result from functional changes in the joint and surrounding tissues. Localized hypermobility may be due to training, excessive stretching, trauma, such as surgery or joint dislocation, among others, but it can also be hereditary. Generalized or systemic hypermobility involves more than five joints and is usually hereditary, with significant individual differences based on age, sex, and ethnic background [26]. The most common genetic conditions associated with systemic hypermobility include Ehlers-Danlos, Marfan, and Down Syndrome. Physiotherapists may assess systemic hypermobility with the Beighton Score (Figure 1), which has acceptable interrater reliability but inconclusive validity [27].
Several other commonly used tests lack satisfactory reliability and validity [27]. The Five-Part Questionnaire (Table 1) is the most used questionnaire for adults with conflicting evidence of reliability and validity [27, 28]. Another commonly used test is the Bristol Impact of Hypermobility test, which measures the impact of hypermobility on a person’s life and showed excellent test-retest reliability (download here) [29].
Table 1 – The Five-Point Questionnaire [28]
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
Systemic hypermobility usually does not pose any major problems until puberty. In clinical practice, many teenage girls with EDS experience their pain and major dysfunction within weeks or months after their first menses. Most children are hypermobile, with girls having greater joint mobility than boys. Range of motion increases until adolescence and tapers into adulthood and old age. Since joint mobility in children is far greater than in adults, diagnosing hypermobility in children is challenging as there are no age-related norms for range of motion. An Australian study of 1,584 subjects showed that 61% of girls and 37% of boys were hypermobile [30]. This does not necessarily indicate that these children had abnormal range of motion or experienced disproportionate pain or dysfunction. Javadi Parvaneh and Shiari (2016) modified the Beighton criteria to improve the identification of hypermobility in children [31], but this test is not commonly used as most children with hypermobility are not symptomatic.
It is important to realize that not all hypermobile people are symptomatic [27]. However, those with systemic hypermobility tend to have higher reports of pain. A study of 466 subjects with EDS demonstrated that 99% suffered from joint pain, 91% suffered from extremity pain, and many common comorbidities, such as chronic fatigue (82%), anxiety (73%), and depression (69%) [32]. Joint hypermobility was a common precursor to pain hypersensitivity and central sensitization in 40 hypermobile adolescents [33].
Ehlers Danlos Syndrome and Hypermobility Spectrum Disorders
As summarized in the 2017 EDS clinical classifications, Ehlers-Danlos Syndromes are a heterogeneous group of thirteen different but overlapping connective tissue disorders featuring joint hypermobility, skin hyperextensibility, and fragile tissues (Table 2) [24]. Many patients with EDS experience persistent pain, autonomic dysfunction, and gastrointestinal dysmotility [34]. Each subtype has its own specific major and minor criteria. For all subtypes, except the hypermobile subtype, the definitive diagnosis must be made by molecular confirmation with identification of the involved gene(s), which implies that the diagnosis of hypermobile EDS is made based on history, examination, and a clinical impression [24].
Table 2 – Clinical Classification of the Ehlers-Danlos Syndromes, Genetic Basis, and Protein Type [24]
Clinical Subtype |
Genetic basis |
Protein |
Classical EDS |
Major: COL5A1, COL5A1Rare: COL1A1 |
Type V collagenType I collagen |
Classical-like EDS |
TNXB |
Tenascin XB |
Cardiac-valvular EDS |
COL1A2 |
Type I collagen |
Vascular EDS |
Major: COL3A1Rare: COL1A1 |
Type III collagenType I collagen |
Hypermobile EDS |
Unknown |
Unknown |
Arthrochalasia EDS |
COL1A1, COL1A2 |
Type I collagen |
Dermatosparaxis EDS |
ADAMTS2 |
ADAMTS2 |
Kyphoscoliotic EDS |
PLOD1FKBP14 |
LH1FKBP22 |
Brittle Cornea syndrome |
ZNF469PRDM5 |
ZNF469PRDM5 |
Spondylodysplastic EDS |
B4GALT7B3GALT6SLC39A13 |
B4GalT7B3GalT6ZIP13 |
Musculocontractural EDS |
CHST14DSE |
D4ST1DSE |
Myopathic EDS |
COL12A1 |
Type XII collagen |
Periodontal EDS |
C1RC1S |
C1rC1s |
Several patients with hypermobility do not meet any of the new criteria, and for these individuals, the term Hypermobility Spectrum Disorders (HSD) was coined [24]. Patients with HSD usually have musculoskeletal symptoms, although some may have limited multisystem involvement. Both hEDS and HSD patients feature a myofibroblast-like phenotype with several abnormal extracellular matrix components (ECM), such as different expressions of CCN1/CYR61 and CCN2/CTGF inflammation mediators, an altered organization of α-smooth muscle actin cytoskeleton, and increased levels of the ECM-degrading metallo-proteinase-9, among others [35]. HSDs are divided into generalized (G-HSD), peripheral (P-HSD), localized (L-HSD), and historical (H-HSD) subtypes. The genetic basis, prevalence, and incidence of HSDs are not known.
Historically, EDS and HSD have been poorly recognized by healthcare providers. In 2005, only 10% of physicians referring their hypermobile EDS patients to rheumatology clinics realized that joint hypermobility was the underlying cause of their patients’ pain [36]. Many physiotherapists are unaware of the condition [37], although EDS is a common, heritable trait seen in up to 10%–30% of males and 20%-40% of females [38]. Ehlers Danlos Syndrome is the most common inherited connective tissue disorder with approximately 1 in 5000 births [39], compared to 1 in 3000-10000 for Marfan Syndrome [40], and 1 in 10000 -15000 for Osteogenesis Imperfecta [41, 42]. The hypermobile type of EDS is characterized not only by hypermobility but also by chronic pain, dysautonomia, chronic fatigue, anxiety, and other associated symptoms and represents at least 80%-90% of all EDS cases [39]. Many patients with hypermobile EDS experience significant levels of disability, which is highly correlated with both physical and psychological factors [43].
As part of the differential diagnostic process, patients must be screened for possible neurological complications resulting from tissue weakness, biophysical deformative stresses, entrapments, and tissue deformations [44]. Comorbid conditions reported with hypermobile EDS include Chiari malformations with or without tethered cord syndrome [45] and craniocervical instability with or without ventral brainstem compression [46]. Other common comorbidities include mast cell activation syndrome [47], gastrointestinal dysfunction [48], and postural orthostatic tachycardia syndrome (POTS) [49].
Dry Needling
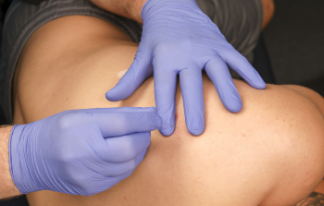
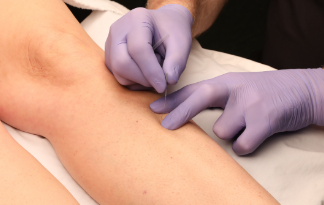
 Since pain is very common in patients with EDS and HSD, DN may seem like an excellent treatment option, but some caution is warranted. Managing pain in hypermobile patients can be quite challenging and often requires a multimodal, interdisciplinary approach [50, 51] that may include manual therapy, such as trigger point therapy and soft tissue mobilizations [52], pain science education [53], cognitive, emotional, and behavioral therapy [54], and external-focus exercise therapy [55, 56]. The role of DN for hypermobile patients has not been scientifically confirmed. Still, based on our extensive clinical experience with hypermobile patients, DN can be an important aspect of physiotherapy for patients with HSD and EDS, especially for reducing pain [57]. Our physiotherapy center, Bethesda Physiocare (Bethesda, Maryland, USA), is one of only 22 centers globally recognized as EDS Centers & Networks of Excellence (Figure 2). We have treated thousands of patients with EDS/HSD.
Since pain is very common in patients with EDS and HSD, DN may seem like an excellent treatment option, but some caution is warranted. Managing pain in hypermobile patients can be quite challenging and often requires a multimodal, interdisciplinary approach [50, 51] that may include manual therapy, such as trigger point therapy and soft tissue mobilizations [52], pain science education [53], cognitive, emotional, and behavioral therapy [54], and external-focus exercise therapy [55, 56]. The role of DN for hypermobile patients has not been scientifically confirmed. Still, based on our extensive clinical experience with hypermobile patients, DN can be an important aspect of physiotherapy for patients with HSD and EDS, especially for reducing pain [57]. Our physiotherapy center, Bethesda Physiocare (Bethesda, Maryland, USA), is one of only 22 centers globally recognized as EDS Centers & Networks of Excellence (Figure 2). We have treated thousands of patients with EDS/HSD.
Trigger point injections were recommended in a case report from India [58]. Studies comparing TrP injections with DN show many similarities in outcomes, but these studies are usually non-pragmatic with limited clinical applicability and methodological flaws [59-64]. As mentioned in the introduction, there is a risk that DN may reduce the patient’s joint stability, especially when that patient uses contractures for stabilization. Because it is sometimes difficult to predict how a patient with EDS will respond to DN, we recommend treating only a few muscles or maybe just a few TrP points during the first treatment sessions combined with pain science education [65-67]. Once a patient’s pain level has reduced, therapy must include progressive or graded loading, improving loading tolerance, and reducing kinesiophobia [68-70]. Another question is whether physiotherapists should needle stiffer areas, for example, sections of the spine, in hypermobile individuals. The stiffer areas may be more “normal” or functional, yet the relatively stiffer areas are also common causes of myofascial pain [57].
Another aspect to consider with needling patients with EDS is that connective tissues, especially fascia, are organized differently than in patients without hypermobility [71]. In hypermobile EDS patients, the extracellular matrix changes exhibit reduced inter-fascial plane gliding [72], which is a bit surprising as, previously, decreased fascial gliding was primarily associated with a loss of flexibility [73]. Type I collagen and hyaluronan are important for fascial force transmission and gliding, promoting sliding of adjacent fascial tissue layers [74], but excessive hyaluronan can lead to stiffness of the ECM [75]. Hyaluronan or hyaluronic acid is a key component of the ECM found in vertebrate tissues [76], including in articular joint synovial fluid, providing lubrication and viscoelasticity to protect cartilage surfaces. The reduced gliding ability found in EDS patients may explain at least partially the widespread pain, but it may also impact proprioception and the ability to generate force and contract muscles efficiently [76, 77].
Up to 40% of muscle force is transmitted via fascia, especially the epimysium, and not through tendons [78, 79]. When connective tissues have significant laxity, forces may not get transmitted to adjacent fascial layers [57]. The changes in fascial gliding may be due to an increase in type I collagen and pathologic changes in matrix metalloproteinases in the ECM [80], which likely results in increased viscosity, reduced lubrication, and sliding movement of fascia [76]. It is conceivable that fascial needling approaches, including winding fascial needles in fascial tissues [81, 82], or Fu Subcutaneous Needling [83-85], may benefit patients with EDS. The theoretical basis for using needles in the treatment of fascial adhesions and scar tissue has developed sufficiently to consider its use in the clinic [86-91], and conceivably, these techniques may also be useful for patients with EDS, but there is no research to support this notion.
As hypermobile patients get older, they will likely become less hypermobile, mostly because the percentage area of collagen 1 increases significantly with aging [92]. There is some evidence that part of aging is associated with a decrease in the percentage of elastic fibers in the perimysium and a decrease in hyaluronan [92]. Older skin has less hyaluronan than younger skin [93]. Another contributing factor is the Yes-associated protein (YAP), expressed particularly in the deep fascia, where it is involved with fascial mechanotransduction, remodeling, regeneration, and fibrogenesis [94].
Dry Needling Course Series
The Dry Needling 1 course is an excellent starting point for learning the fundamentals of dry needling therapy. With a focus on the safe and effective application of dry needling techniques, you will gain a solid understanding of myofascial trigger points, needling techniques, precautions, and how to apply these techniques in clinical practice.
The Dry Needling 2 course is an intermediate-level course that provides in-depth knowledge and hands-on training for dry needling techniques of the extremities, including the upper and lower body. By completing this course, you will expand upon the skills you acquired in the DN-1 course and better understand the application of dry needling for managing musculoskeletal pain and dysfunction.
The Dry Needling 3 course is the final course in the series and the last step before becoming a Certified Myofascial Trigger Point Therapist – Dry Needling (CMTPT/DN). This course offers an in-depth study of advanced dry needling techniques for hand muscles, several lower extremity and foot muscles, the craniofacial and craniomandibular muscles, and more.
References
- Hernández-Secorún, M., et al., Effectiveness of Dry Needling in Improving Pain and Function in Comparison with Other Techniques in Patients with Chronic Neck Pain: A Systematic Review and Meta-Analysis. Pain Res Manag, 2023. 2023: p. 1523834.
- Hernández-Secorún, M., et al., Effectiveness of Dry Needling in Improving Pain and Function in Comparison with Other Techniques in Patients with Chronic Neck Pain: A Systematic Review and Meta-Analysis. Pain Research and Management, 2023. 2023: p. 1523834.
- Forogh, B., et al., Efficacy of trigger point dry needling on pain and function of the hip joint: a systematic review of randomized clinical trials. Acupunct Med, 2023: p. 9645284231207870.
- Vázquez-Justes, D., et al., Effectiveness of dry needling for headache: A systematic review. Neurología (English Edition), 2022. 37(9): p. 806-815.
- Valera-Calero, J.A., et al., Efficacy of Dry Needling and Acupuncture in Patients with Fibromyalgia: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health, 2022. 19(16).
- Rodríguez-Huguet, M., M.J. Vinolo-Gil, and J. Góngora-Rodríguez, Dry Needling in Physical Therapy Treatment of Chronic Neck Pain: Systematic Review. J Clin Med, 2022. 11(9).
- Nuhmani, S., et al., Dry needling in the management of tendinopathy: A systematic review of randomized control trials. Journal of Bodywork and Movement Therapies, 2022.
- Jiménez-Del-Barrio, S., et al., The Effectiveness of Dry Needling in Patients with Hip or Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Life (Basel), 2022. 12(10).
- Javier-Ormazábal, A., et al., Invasive Physiotherapy as a Treatment of Spasticity: A Systematic Review. Degener Neurol Neuromuscul Dis, 2022. 12: p. 23-29.
- Griswold, D., et al., Dry Needling for Subacromial Pain Syndrome. A Systematic Review with Meta-analysis. Pain Med, 2022.
- Giorgi, E., et al., The Effectiveness of Dry Needling Combined With Therapeutic Exercises in Treating Tendinopathy Conditions: A Systematic Review. J Sport Rehabil, 2022. 31(7): p. 918-924.
- Blanco-Díaz, M., et al., A Systematic Review of the Effectiveness of Dry Needling in Subacromial Syndrome. Biology, 2022. 11(2): p. 243.
- Ughreja, R.A. and V. Prem, Effectiveness of dry needling techniques in patients with knee osteoarthritis: A systematic review and meta-analysis. J Bodyw Mov Ther, 2021. 27: p. 328-338.
- Pourahmadi, M., et al., Dry needling for the treatment of tension-type, cervicogenic, or migraine headaches: a systematic review and meta-analysis. Phys Ther, 2021. 101(5).
- Hadizadeh, M., et al., The efficacy of intramuscular electrical stimulation in the management of patients with myofascial pain syndrome: a systematic review. Chiropractic and Manual Therapies, 2021. 29(1).
- Navarro-Santana, M.J., et al., Effects of Trigger Point Dry Needling for Nontraumatic Shoulder Pain of Musculoskeletal Origin: A Systematic Review and Meta-Analysis. Physical Therapy, 2021. 101(2): p. pzaa216.
- Navarro-Santana, M.J., et al., Effectiveness of Dry Needling for Myofascial Trigger Points Associated with Neck Pain Symptoms: An Updated Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 2020. 9(10).
- Navarro-Santana, M.J., et al., Effects of trigger point dry needling on lateral epicondylalgia of musculoskeletal origin: a systematic review and meta-analysis. Clin Rehabil, 2020. 34(11): p. 1327-1340.
- Sarmiento-Hernández, I., et al., Effectiveness of Invasive Techniques in Patients with Fibromyalgia: Systematic Review and Meta-Analysis. Pain Medicine, 2020. 21(12): p. 3499-3511.
- Fernandez-de-Las-Penas, C., et al., Is Dry Needling Effective for the Management of Spasticity, Pain, and Motor Function in Post-Stroke Patients? A Systematic Review and Meta-Analysis. Pain Med, 2021. 22(1): p. 131-141.
- Fernandez-De-Las-Penas, C., et al., Is Dry Needling Effective When Combined with Other Therapies for Myofascial Trigger Points Associated with Neck Pain Symptoms? A Systematic Review and Meta-Analysis. Pain Res Manag, 2021. 2021: p. 8836427.
- Soucie, J.M., et al., Range of motion measurements: reference values and a database for comparison studies. Haemophilia, 2011. 17(3): p. 500-7.
- Keer, R. and J. Simmonds, Joint protection and physical rehabilitation of the adult with hypermobility syndrome. Curr Opin Rheumatol, 2011. 23(2): p. 131-6.
- Malfait, F., et al., The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet, 2017. 175(1): p. 8-26.
- Dommerholt, J. and N. Mayberry, Hypo- and Hypermobility, in Fascia in Sport and Movement, R. Schleip, J. Wilke, and A. Baker, Editors. 2021, Handspring Publishing: Pencaitland. p. 77-95.
- Coles, W., A. Copeman, and K. Davies, Hypermobility in children. Paediatric Child Health 2017. 28(2): p. 50-56.
- Juul-Kristensen, B., et al., Measurement properties of clinical assessment methods for classifying generalized joint hypermobility-A systematic review. Am J Med Genet C Semin Med Genet, 2017. 175(1): p. 116-147.
- Hakim, A.J. and R. Grahame, A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int J Clin Pract, 2003. 57(3): p. 163-6.
- Palmer, S., et al., Test-retest reliability and smallest detectable change of the Bristol Impact of Hypermobility (BIoH) questionnaire. Musculoskelet Sci Pract, 2017. 32: p. 64-69.
- Morris, S.L., et al., Hypermobility and Musculoskeletal Pain in Adolescents. J Pediatr, 2017. 181: p. 213-221 e1.
- Javadi Parvaneh, V. and R. Shiari, Proposed modifications to Beighton criteria for the diagnosis of joint hypermobility in children. Indian J Rheumatol, 2016. 11: p. 97-100.
- Murray, B., et al., Ehlers-Danlos syndrome, hypermobility type: A characterization of the patients’ lived experience. Am J Med Genet A, 2013. 161A(12): p. 2981-8.
- Bettini, E.A., et al., Association between Pain Sensitivity, Central Sensitization, and Functional Disability in Adolescents With Joint Hypermobility. J Pediatr Nurs, 2018. 42: p. 34-38.
- Beckers, A.B., et al., Gastrointestinal disorders in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type: A review for the gastroenterologist. Neurogastroenterol Motil, 2017. 29(8).
- Zoppi, N., et al., Dermal fibroblast-to-myofibroblast transition sustained by alphavss3 integrin-ILK-Snail1/Slug signaling is a common feature for hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Biochim Biophys Acta Mol Basis Dis, 2018. 1864(4 Pt A): p. 1010-1023.
- Adib, N., et al., Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology, 2005. 44(6): p. 744-50.
- Palmer, S., et al., Physiotherapy management of joint hypermobility syndrome–a focus group study of patient and health professional perspectives. Physiotherapy, 2016. 102(1): p. 93-102.
- Hakim, A. and R. Grahame, Joint hypermobility. Best Pract Res Clin Rheumatol, 2003. 17(6): p. 989-1004.
- Tinkle, B., et al., Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. Am J Med Genet C Semin Med Genet, 2017. 175(1): p. 48-69.
- Pyeritz, R.E., Etiology and pathogenesis of the Marfan syndrome: current understanding. Ann Cardiothorac Surg, 2017. 6(6): p. 595-598.
- Lafage-Proust, M.H. and I. Courtois, The management of Osteogenesis Imperfecta in Adults: state of the art. Joint Bone Spine, 2019. 2019: p. in press.
- Morello, R., Osteogenesis imperfecta and therapeutics. Matrix Biol, 2018. 71-72: p. 294-312.
- Scheper, M.C., et al., Disability in Adolescents and Adults Diagnosed With Hypermobility-Related Disorders: A Meta-Analysis. Arch Phys Med Rehabil, 2016. 97(12): p. 2174-2187.
- Henderson, F.C., Sr., et al., Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet, 2017. 175(1): p. 195-211.
- Milhorat, T.H., et al., Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine, 2007. 7(6): p. 601-9.
- Henderson, F.C., Sr., et al., Utility of the clivo-axial angle in assessing brainstem deformity: pilot study and literature review. Neurosurg Rev, 2018. 41(1): p. 149-163.
- Seneviratne, S.L., A. Maitland, and L. Afrin, Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet, 2017. 175(1): p. 226-236.
- Botrus, G., et al., Spectrum of Gastrointestinal Manifestations in Joint Hypermobility Syndromes. Am J Med Sci, 2018. 355(6): p. 573-580.
- Bonamichi-Santos, R., et al., Association of Postural Tachycardia Syndrome and Ehlers-Danlos Syndrome with Mast Cell Activation Disorders. Immunol Allergy Clin North Am, 2018. 38(3): p. 497-504.
- Revivo, G., et al., Interdisciplinary Pain Management Improves Pain and Function in Pediatric Patients with Chronic Pain Associated with Joint Hypermobility Syndrome. PM R, 2018.
- Zhou, Z., A. Rewari, and H. Shanthanna, Management of chronic pain in Ehlers-Danlos syndrome: Two case reports and a review of literature. Medicine (Baltimore), 2018. 97(45): p. e13115.
- Tewari, S., et al., Chronic pain in a patient with Ehlers-Danlos syndrome (hypermobility type): The role of myofascial trigger point injections. J Bodyw Mov Ther, 2017. 21(1): p. 194-196.
- Louw, A., Treating the brain in chronic pain, in Manual therapy for musculoskeletal pain syndromes – an evidenced and clinical-informed approach, C. Fernández de las Peñas, J. Cleland, and J. Dommerholt, Editors. 2016, Churchill Livingstone (Elsevier): Edinburgh.
- Baeza-Velasco, C., et al., Cognitive, emotional, and behavioral considerations for chronic pain management in the Ehlers-Danlos syndrome hypermobility-type: a narrative review. Disabil Rehabil, 2018: p. 1-9.
- Wulf, G. and R. Lewthwaite, Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon Bull Rev, 2016. 23(5): p. 1382-1414.
- Wulf, G., et al., Triple play: Additive contributions of enhanced expectancies, autonomy support, and external attentional focus to motor learning. Q J Exp Psychol (Hove), 2018. 71(4): p. 824-831.
- Langevin, H.M., C.A. Francomano, and C. DaPrato, Chapter 5 – Myofascial pain: Myofascial knee pain in a young woman with hypermobile Ehlers-Danlos syndrome, in The Symptom-Based Handbook for Ehlers-Danlos Syndromes and Hypermobility Spectrum Disorders, C.A. Francomano, et al., Editors. 2024, Elsevier. p. 45-49.
- Tewari, S., et al., Chronic pain in a patient with Ehlers-Danlos syndrome (hypermobility type): The role of myofascial trigger point injections. Journal of Bodywork and Movement Therapies, 2017. 21(1): p. 194-196.
- Cummings, T.M. and A.R. White, Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil, 2001. 82(7): p. 986-92.
- Kara, S., et al., Comparison of kinesio taping, dry needling and lidocaine injection methods in myofascial pain syndrome. Journal of Bodywork and Movement Therapies, 2024. 38: p. 128-132.
- Griswold, D., et al., Comparing dry needling or local acupuncture to various wet needling injection types for musculoskeletal pain and disability. A systematic review of randomized clinical trials. Disability and Rehabilitation, 2023: p. 1-15.
- Navarro-Santana, M.J., et al., Dry Needling Versus Trigger Point Injection for Neck Pain Symptoms Associated with Myofascial Trigger Points: A Systematic Review and Meta-Analysis. Pain Med, 2022. 23(3): p. 515-525.
- Nagarajan, V., et al., Local Corticosteroid Injection Versus Dry Needling in the Treatment of Lateral Epicondylitis. Cureus, 2022. 14(11): p. e31286.
- Nowak, Z., et al., Intramuscular injections and dry needling within masticatory muscles in management of myofascial Pain. Systematic review of clinical trials. Int J Environ Res Public Health, 2021. 18(18).
- Diener, I., M. Kargela, and A. Louw, Listening is therapy: Patient interviewing from a pain science perspective. Physiother Theory Pract, 2016. 32(5): p. 356-67.
- Bonatesta, L., et al., Pain Science Education Plus Exercise Therapy in Chronic Nonspecific Spinal Pain: A Systematic Review and Meta-analyses of Randomized Clinical Trials. J Pain, 2022. 23(4): p. 535-546.
- Chimenti, R.L., et al., The effects of pain science education plus exercise on pain and function in chronic Achilles tendinopathy: a blinded, placebo-controlled, explanatory, randomized trial. Pain, 2023. 164(1): p. e47-e65.
- Vaegter, H.B., et al., Kinesiophobia is associated with pain intensity but not pain sensitivity before and after exercise: an explorative analysis. Physiotherapy, 2018. 104(2): p. 187-193.
- Priore, L.B., et al., Influence of kinesiophobia and pain catastrophism on objective function in women with patellofemoral pain. Phys Ther Sport, 2019. 35: p. 116-121.
- Dommerholt, J., N. Mayberry, and M. Sickel, Physiotherapie mit externem Fokus. Der Schmerzpatient, 2019. 2(4): p. 178-187.
- Wang, T.J. and A. Stecco, Fascial thickness and stiffness in hypermobile Ehlers-Danlos syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 2021. 187(4): p. 446-452.
- Wang, T.J., et al., Change in gliding properties of the iliotibial tract in hypermobile Ehlers–Danlos Syndrome. Journal of Ultrasound, 2023. 26(4): p. 809-813.
- Cruz-Montecinos, C., et al., In vivo relationship between pelvis motion and deep fascia displacement of the medial gastrocnemius: anatomical and functional implications. Journal of Anatomy, 2015. 227(5): p. 665-672.
- Fede, C., et al., A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int J Mol Sci, 2021. 22(3).
- Mambetsariev, N., et al., Hyaluronic Acid Binding Protein 2 Is a Novel Regulator of Vascular Integrity. Arteriosclerosis, Thrombosis, and Vascular Biology, 2010. 30(3): p. 483-490.
- Cowman, M.K., et al., The Content and Size of Hyaluronan in Biological Fluids and Tissues. Frontiers in Immunology, 2015. 6.
- Stecco, C., et al., The Ankle Retinacula: Morphological Evidence of the Proprioceptive Role of the Fascial System. Cells Tissues Organs, 2010. 192(3): p. 200-210.
- Yucesoy, C.A., et al., Extramuscular Myofascial Force Transmission: Experiments and Finite Element Modeling. Archives of Physiology and Biochemistry, 2003. 111(4): p. 377-388.
- Huijing, P.A., Epimuscular myofascial force transmission: a historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei, 2007. J Biomech, 2009. 42(1): p. 9-21.
- Chiarelli, N., et al., Matrix Metalloproteinases Inhibition by Doxycycline Rescues Extracellular Matrix Organization and Partly Reverts Myofibroblast Differentiation in Hypermobile Ehlers-Danlos Syndrome Dermal Fibroblasts: A Potential Therapeutic Target? Cells, 2021. 10(11): p. 3236.
- Langevin, H.M., et al., Connective tissue fibroblast response to acupuncture: dose-dependent effect of bidirectional needle rotation. J Altern Complement Med, 2007. 13(3): p. 355-60.
- Langevin, H.M., et al., Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: Evidence for a mechanotransduction-based mechanism. J Cell Physiol, 2006. 207(3): p. 767-74.
- Xiao, A.J., et al., [Review on the role of Fu’s subcutaneous needling (FSN) in pain relieving]. Zhongguo Zhen Jiu, 2013. 33(12): p. 1143-6.
- Xu, W., Further discussion on the effect of FSN to immune system. j Chin Med Acupunct, 2020. 27(1): p. 1-5.
- Huang, C.H., et al., Efficacy of Fu’s Subcutaneous Needling on Myofascial Trigger Points for Lateral Epicondylalgia: A Randomized Control Trial. Evid Based Complement Alternat Med, 2022. 2022: p. 5951327.
- Rozenfeld, E., et al., Dry needling for scar treatment. Acupunct Med, 2020. 38(6): p. 435-439.
- Chiquet, M., et al., How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol, 2003. 22(1): p. 73-80.
- Finando, S. and D. Finando, Fascia and the mechanism of acupuncture. J Bodyw Mov Ther, 2011. 15(2): p. 168-76.
- Grinnell, F., Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol, 2003. 13(5): p. 264-9.
- Langevin, H.M., et al., Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J Cell Physiol, 2011. 226(5): p. 1166-75.
- Pirri, C., et al., Elastic Fibres in the subcutaneous tissue: Is there a difference between superficial and muscular fascia? A cadaver study. Skin Res Technol, 2022. 28(1): p. 21-27.
- Fede, C., et al., The Effects of Aging on the Intramuscular Connective Tissue. International Journal of Molecular Sciences, 2022. 23(19): p. 11061.
- Laurent, U.B.G. and A. Tengblad, Determination of hyaluronate in biological samples by a specific radioassay technique. Analytical Biochemistry, 1980. 109(2): p. 386-394.
- Pirri, C., et al., A New Player in the Mechanobiology of Deep Fascia: Yes-Associated Protein (YAP). International Journal of Molecular Sciences, 2023. 24(20): p. 15389.